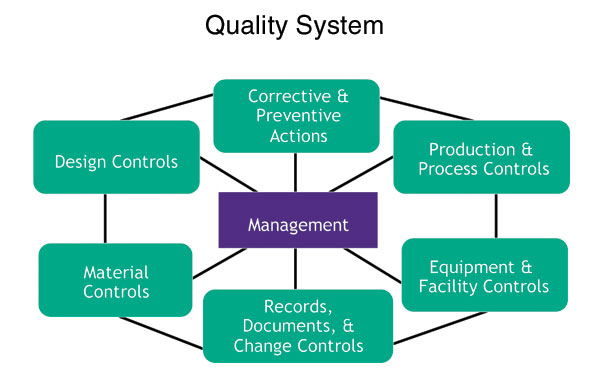
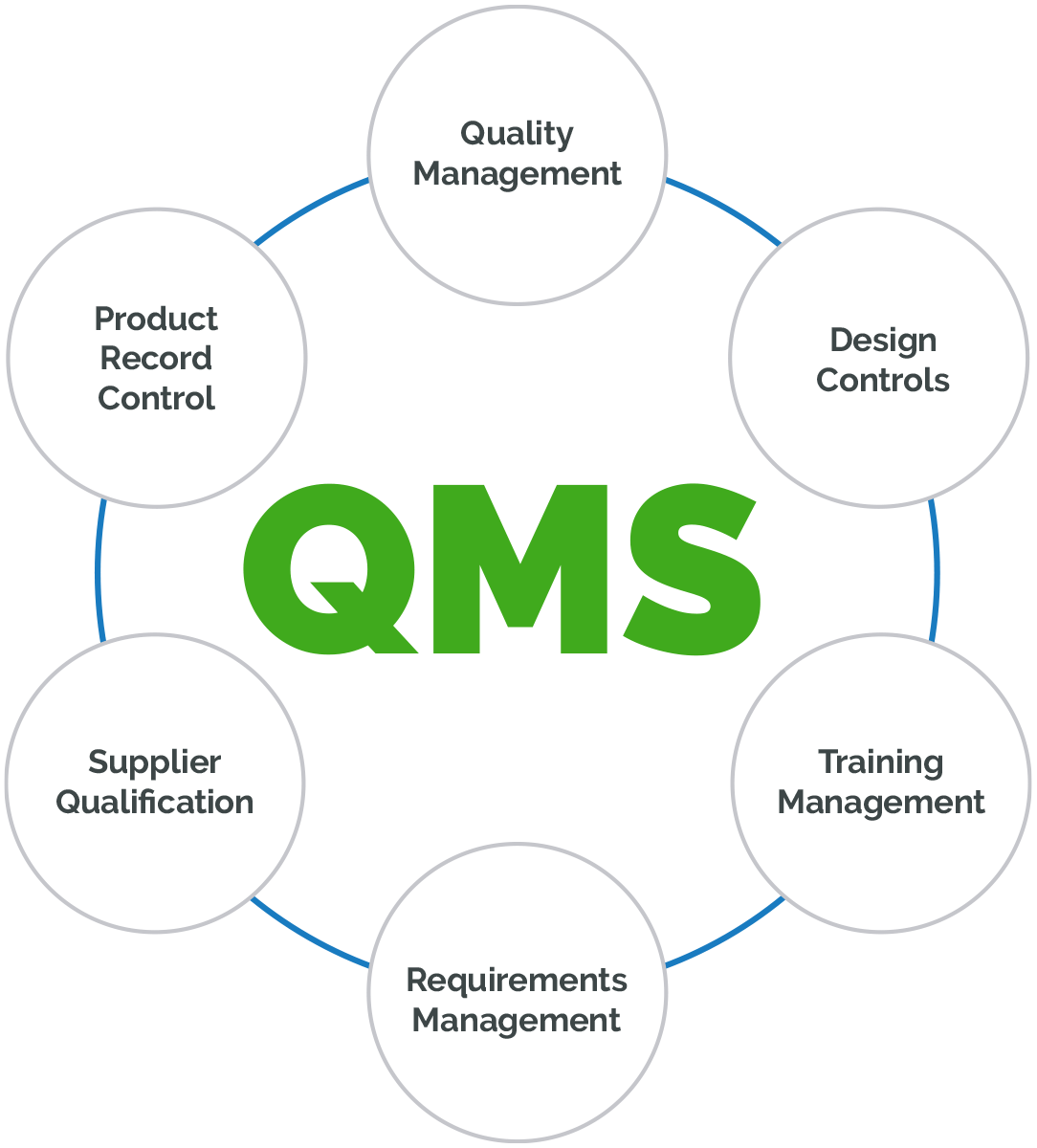
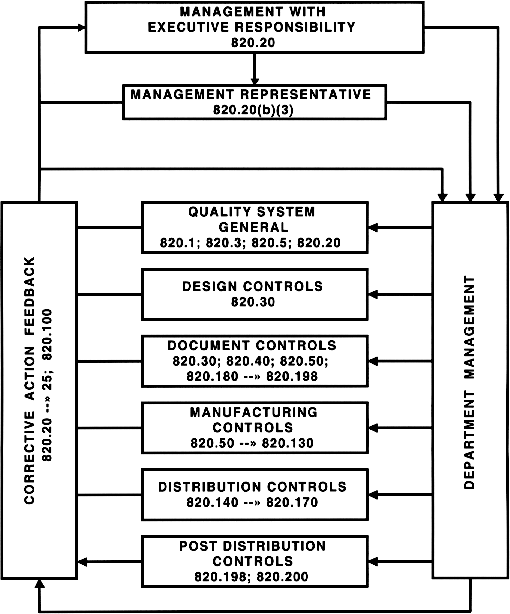
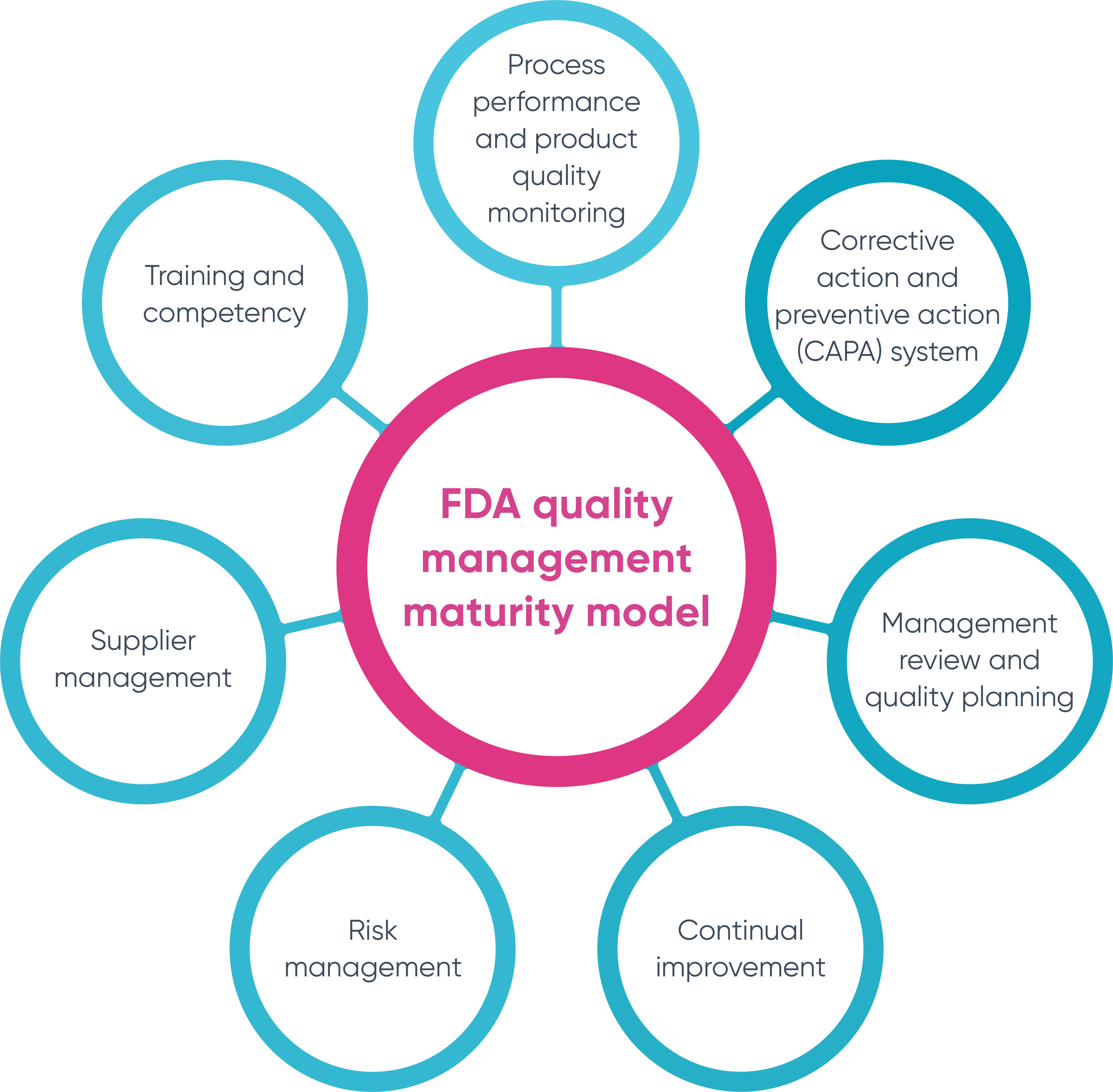
Designing A World-Class Quality Management System For FDA Regulated Industries: Quality System Requirements (QSR) For cGMP : Muchemu, David: Amazon.fr: Livres

The FDA publishes its Quality Management System Regulation (QMSR) final Rule to harmonize with ISO13485 - Evnia

New FDA Quality Management System Regulation (QMSR): Why Medical Device Manufacturers Need to Prepare Now - Johner Institute New Zealand

End-to-End Biologics CDMO Quality Management System|OPM Biosciences – Cell Culture Media and End-to-End CDMO