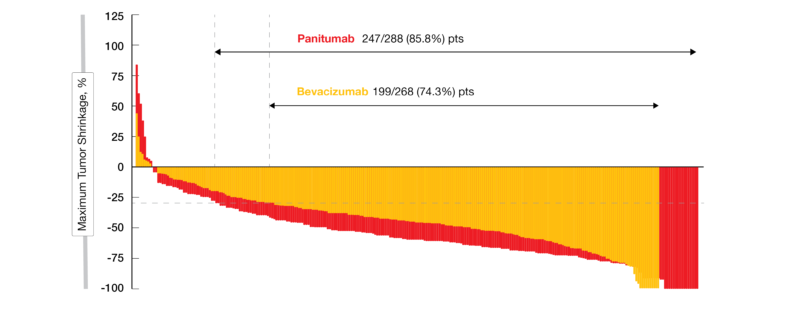
World-first confirmation of standard treatment for RAS wild-type colorectal cancer -Paper published in JAMA- | National Cancer Center Japan

Seyda Gunduz on X: "#PARADIGM trial First-line Panitumab is superior to bevacizumab in the left-sided RAS wild metastatic colon cancer #ASCO22 https://t.co/6XLAWIK1c2" / X
ASCO GI 2023 Biomarker Study of The Phase III PARADIGM Trial: Negative Hyperselection of Patients With RAS WT mCRC for Panitumumab | VuMedi

Therapeutic landscape and future direction of metastatic colorectal cancer | Nature Reviews Gastroenterology & Hepatology

Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort | Research Communities by Springer Nature
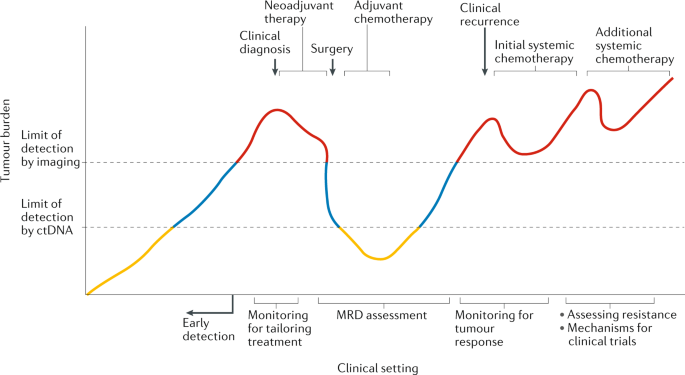
ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal–Anal Task Forces whitepaper | Nature Reviews Clinical Oncology
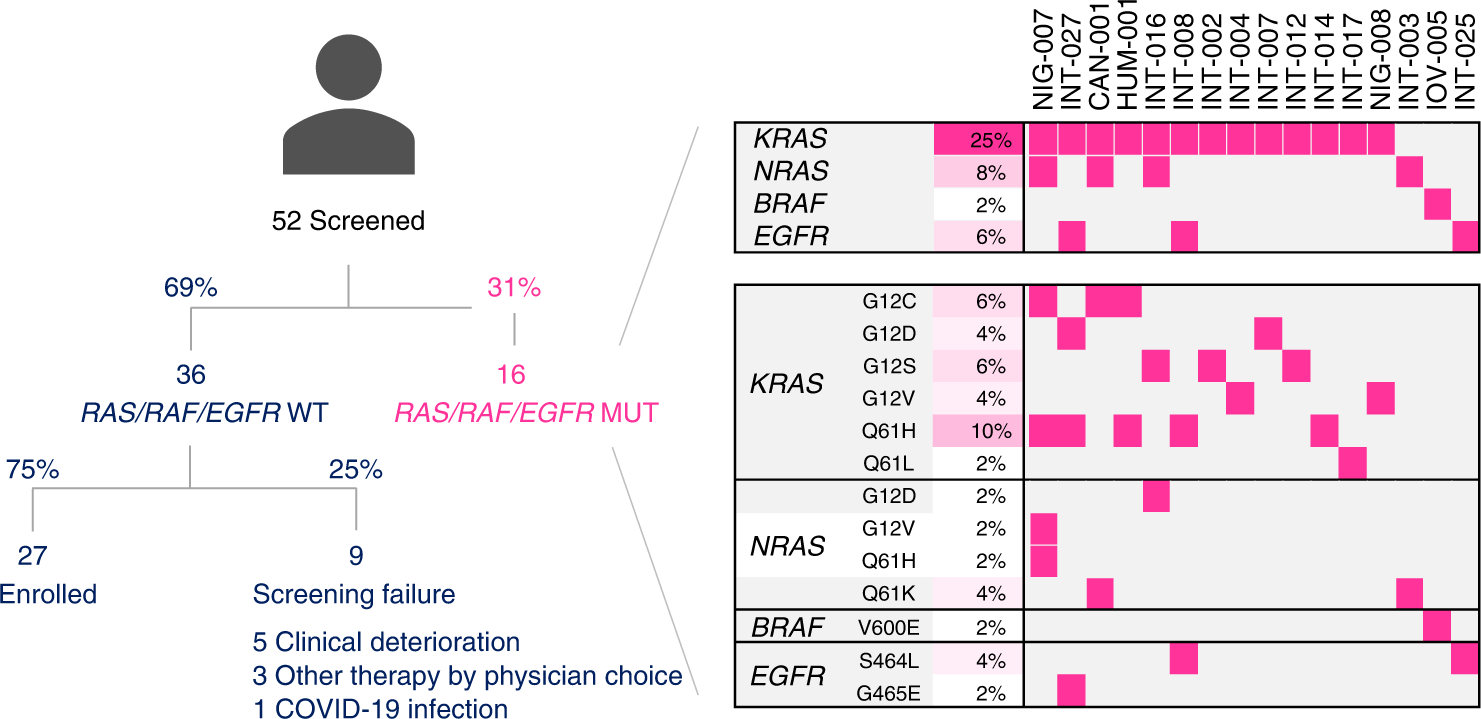
Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial | Nature Medicine
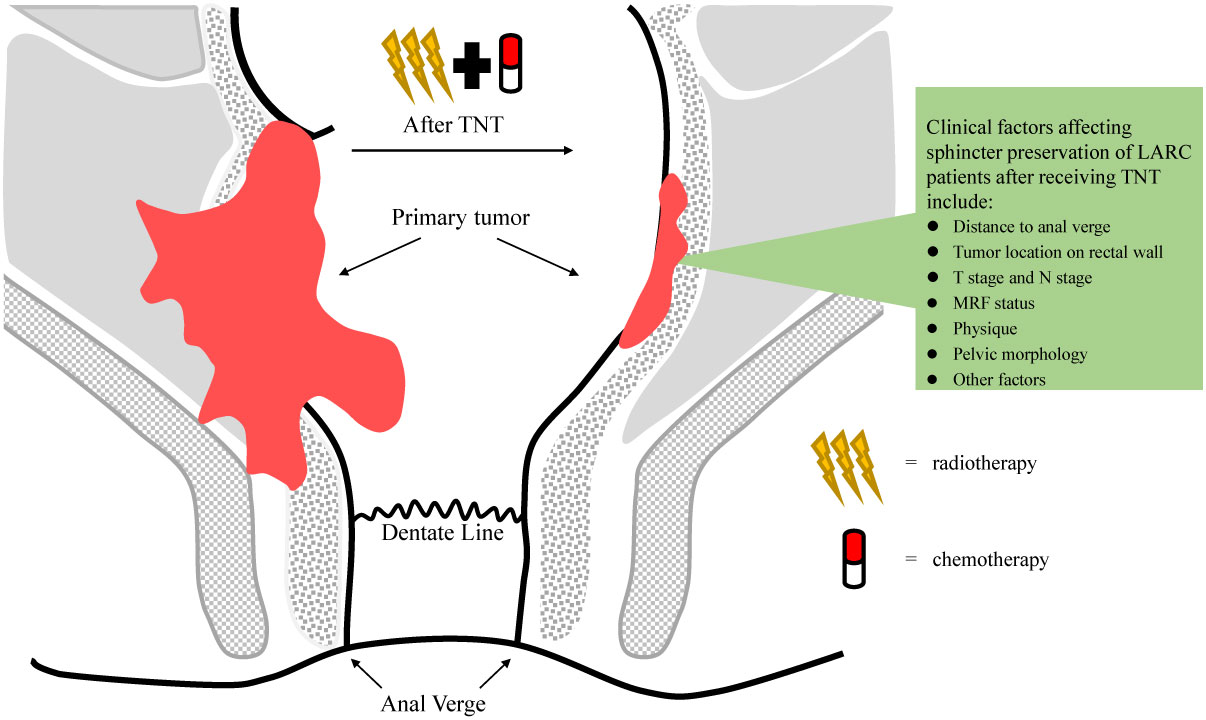
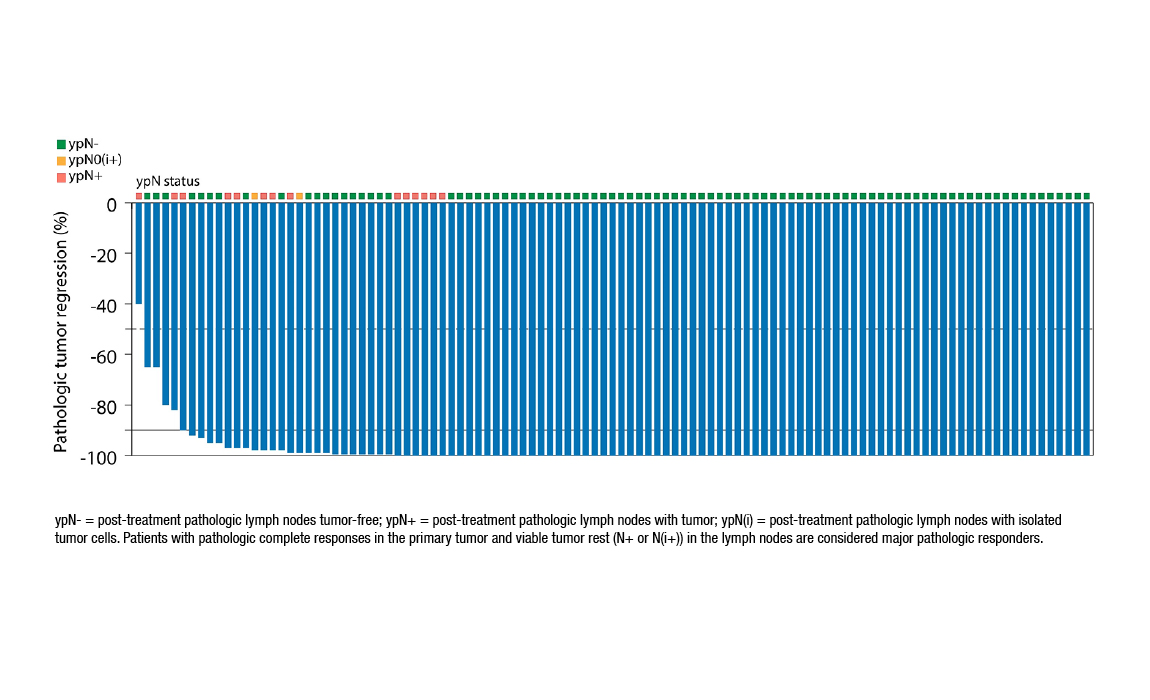
Frontiers | Total neoadjuvant treatment and PD-1/PD-L1 checkpoint inhibitor in locally advanced rectal cancer
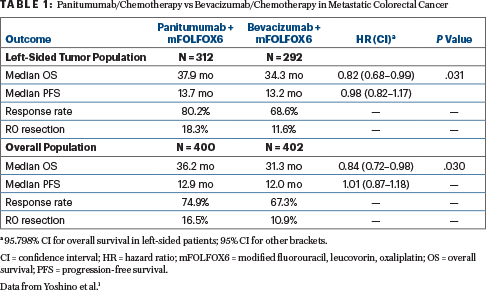
In Metastatic RAS Wild-Type Left-Sided Colorectal Cancer, Panitumumab Proves Superior to Bevacizumab - The ASCO Post

Total Neoadjuvant Therapy: A Shifting Paradigm in Locally Advanced Rectal Cancer Management - ScienceDirect
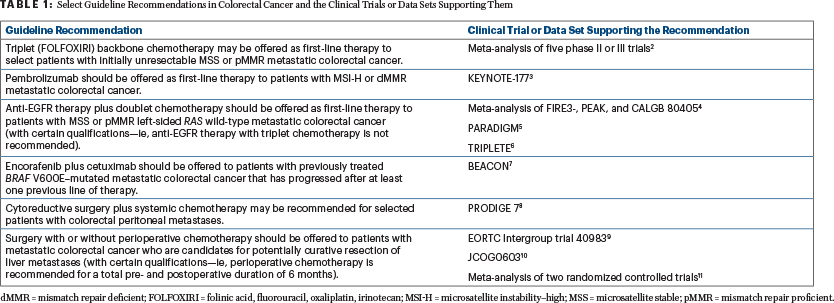
ASCO Guideline Highlights Newest Breakthroughs in the Treatment of Metastatic Colorectal Cancer - The ASCO Post